You may also be interested to see an interview with Dr. Genevieve Boland, who led this study, which was published in Select Science – view the article here.
Webinar
Some examples of how Olink panels have been used in the field of cancer immunotherapy have been presented in a Science/AAAS webinar, “Emerging liquid biopsy biomarkers: Predicting response and resistance to cancer immunotherapy“.
This was originally broadcast on 9th October 2019, featuring Genevieve Boland, M.D., Ph.D., FACS Massachusetts General Hospital, Boston, MA and Evert Jan Van Limbergen, M.D., Ph.D.Maastro Clinic Maastricht, The Netherlands. Click here to register and access the on-demand recording of these presentations.
Olink in immunotherapy research
Olink’s solutions for protein biomarker discovery (Olink Explore 1536 and Olink Target 96 panels) are being increasingly used in immunotherapy studies across different tumor types and different immunotherapy treatment strategies and settings. These include:
Objectives:
- Predict and monitor treatment response, patient stratification
- Tumor biology insight, mechanism of immune modulation, phenotype changes, transformation cold to hot tumors
- Clarify the interconnected roles of tumor genomics, transcriptomics and proteomics in predicting response to immunotherapy
- Treatment sensitivity and mechanism of immune resistance, design of strategies to optimize response rates and identify novel targetable pathways
- Assess the longitudinal biomarker changes between early timepoints (baseline to treatment) and later timepoints (baseline to disease progression) to identify key markers associated with the transition from a drug-sensitive to drug-resistant state
- Explore the association between baseline circulating immune proteins and patient outcome (OS and PFS)
- Predict onset and mechanism of treatment associated toxicity, monitoring CRS and neurotoxicity
- Predict recurrence and on-treatment progression in adjuvant settings
Cancer types:
Melanoma, NSCLC, Prostate, Pancreatic, Breast, Gastroesophageal, Gastric, Colorectal, Urothelial bladder, Hepatocellular, Lymphoma
Treatment types:
ICB, T cells (CAR-T cells, NK cells), Adenovirus, Cancer vaccines, Bi-specific Ab and PD-1 combinations: Chemo, SBRT, VEGF/R, TKI, Cytokines
Recommended products for immunotherapy applications
What immunotherapy researchers have to say

“Our work with Olink on plasma-based immuno-oncology (IO) applications has allowed the identification of tumor and immune changes associated with immune checkpoint inhibitor response/non-response.This is particularly exciting as we combine this technology with deep tumor-based analysis to understand tumor-immune interactions and establish predictive and/or monitoring tools for IO. The small volume required for Olink analysis is a game-changer as it allows multi-analyte assessment of samples and validation of findings by multiple orthogonal platforms.”
Genevieve M. Boland, Director Melanoma Surgery Program, Massachusetts General Hospital, Boston
More examples of immunotherapy studies using Olink
Collaborative research initiatives
CIMAC-CIDC
The Cancer Immune Monitoring and Analysis Centers-Cancer Immunologic Data Commons (CIMAC-CIDC) Network was launched in 2017 through the Cancer MoonshotSM initiative supported by the NCI, and collaborates with another Cancer Moonshot project, the Partnership for Accelerating Cancer Therapies (PACT). Olink has been chosen as the core assay for profiling of cytokines, chemokines, and growth factors within this project, as reported in a recent article from CIMAC-CIDC/PACT:
Chen et al (2021) Clin. Cancer Res, DOI: 10.1158/1078-0432.CCR-20-3241 – article link
You can also read more about this important initiative for cancer immunotherapy research at the CIMAC-CIDC website
Maastricht University Medical Center
An exploratory prospective study, performed in collaboration with Maastricht University Medical Center, investigated circulating biomarkers of immunogenic cell death (ICD) after radiotherapy and chemotherapy in stage III NSCLC and identified important changes in immune protein profile. The overarching goal in on-going follow up prospective study is to improve therapeutic efficacy of immunotherapy in stage III NSCLC patients treated with concurrent chemotherapy and photon or proton radiotherapy and adjuvant durvalumab (anti-PD-L1). Some of this work will be presented at AACR 2021, in a presentation entitled, “Biomarkers in Radiotherapy” that will be held in the session “Advances in Diagnostics and Therapeutics Session Proton Therapy and FLASH Irradiation” on Monday, April 12, 2021.
“To exploit immunotherapy for cancer optimally, biomarkers are essential for, they will lead to personalized selection of the best treatment during the course of the disease. Blood biomarkers are ideal in this respect as they allow repeated sampling and avoid sampling heterogeneity within the tumor and between tumor locations in the same patient as is the case with tumor biopsies.”
Dr. Dirk de Ruysscher, Professor of Respiratory Oncology, Maastricht University Medical Center, Maastro Clinic, Netherlands
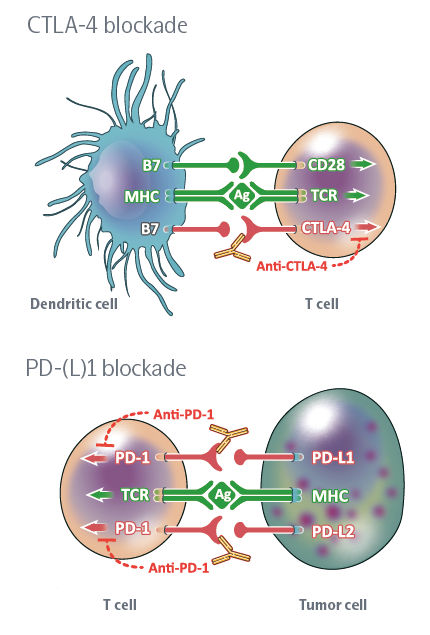 Immune checkpoint targets
Immune checkpoint targets
